|
| | Triphenylmethanol Basic information |
| | Triphenylmethanol Chemical Properties |
| | Triphenylmethanol Usage And Synthesis |
| Chemical Properties | white powder or colorless trisolated crystals. Soluble in alcohol, ether and benzene, dark yellow when dissolved in concentrated sulfuric acid, colorless when dissolved in glacial acetic acid, insoluble in water and petroleum ether. Distilled at 360-380℃ without decomposition. | | Uses | Triphenylmethanol is used as a reagent in the research laboratory. It acts as an intermediate in the production of the commercially useful triarylmethane dyes. It is used in the preparation of triphenylmethane. It is also used as an antiproliferative agent. Further, it is used in the preparation of two-electron reduction product of pyrylogen. In addition to this, it reacts with triphenylphosphine oxide to form a 1:1 molecular complex. It serves as a specific clathrate host for methanol and dimethyl sulphoxide and forms clathrate inclusion complexes.
Triphenylmethanol was used in the synthesis of of the two-electron reduction product of pyrylogen.
It undergoes reduction to triphenylmethane by 9, 10-dihydro-10-methylacridine in the presence of perchloric acid. | | Uses | Triphenylmethanol (Zidovudine EP Impurity D) is a triaryl methane derivative as antiproliferative agent. | | Preparation | Triphenylmethanol synthesis: Triphenylmethanol was prepared by the action of benzene with carbon tetrachloride in the presence of Aluminum chloride, followed by acidification and hydrolysis.
Triphenylmethanol can also be prepared by the reaction of phenylmagnesium bromide with methyl benzoate (instead of benzophenone).
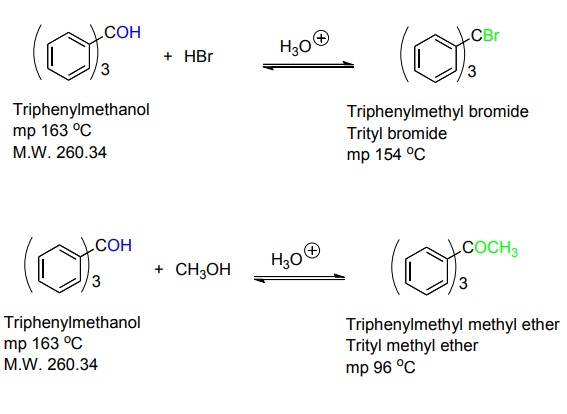
Synthesis of Triphenylmethanol | | Reactions | The first one is the formation of the triphenylmethyl bromide from the reaction of triphenylmethanol with hydrobromic acid.
The second reaction is the formation of an ether from the reaction of triphenylmethanol with methanol in acidic conditions.
| | Synthesis Reference(s) | Organic Syntheses, Coll. Vol. 3, p. 839, 1955
The Journal of Organic Chemistry, 57, p. 4555, 1992 DOI: 10.1021/jo00042a044 | | General Description | Triphenylmethanol forms 1:1 molecular complex with triphenylphosphine oxide. It is a specific clathrate host for methanol and dimethyl sulphoxide and forms clathrate inclusion complexes. It undergoes reduction to triphenylmethane by 9, l0-dihydro-10-methylacridine in the presence of perchloric acid. | | Purification Methods | Crystallise the carbinol from EtOH, MeOH, CCl4 (4mL/g), *benzene, hexane or pet ether (b 60-70o). Dry it at 90o. [Ohwada et al. J Am Chem Soc 108 3029 1986, Beilstein 6 IV 5014.] |
| | Triphenylmethanol Preparation Products And Raw materials |
|



