|
| | lubiprostone Basic information |
| Product Name: | lubiprostone | | Synonyms: | (2R,4aR,5R,7aR)-2-(1,1-Difluoropentyl)-2-hydroxy-6-oxo-3,4,4a,5,7,7a-hexahydrocyclopenta[b]pyran-5-heptanoic acid;Prostan-1-oic acid, 11,15-epoxy-16,16-difluoro-15-hydroxy-9-oxo-, (11alpha,15R)-;Spi 0211;Unii-7662kg2R6k;(2R,4aR,5R,7aR)-2-(1,1-Difluoropentyl)-2-hydroxy-6-oxo-3,4,4a,5,7,7a-hexahydrocyclopenta[b]pyran-5-h;7-((1R,2R,3R)-2-(4,4-difluoro-3-oxooctyl)-3-hydroxy-5-oxocyclopentyl)heptanoic acid;AMitiza Lubiprostone;7-((2R,4aR,5R,7aR)-2-(1,1-difluoropentyl)-2-hydroxy-6-oxooctahydrocyclopenta[b]pyran-5-yl)heptanoic acid | | CAS: | 333963-40-9 | | MF: | C20H32F2O5 | | MW: | 390.46 | | EINECS: | 1312995-182-4 | | Product Categories: | API | | Mol File: | 333963-40-9.mol |  |
| | lubiprostone Chemical Properties |
| Boiling point | 532.3±50.0 °C(Predicted) | | density | 1.175 | | storage temp. | -20°C Freezer | | pka | 4.77±0.10(Predicted) |
| | lubiprostone Usage And Synthesis |
| Description | Chronic constipation is an affliction affecting 4–5 million Americans alone.
When no specific cause is identified, it is classified as idiopathic. Dietary and
lifestyle modifications are the first-line conventional approaches followed by the
administration of laxatives. Unfortunately, chronic idiopathic constipation is frequently
refractory to traditional therapy; thus, the need for novel agents exists.
Lubiprostone is a bicyclic fatty acid with a novel mechanism of action. Without
affecting sodium and potassium ion concentrations, lubiprostone activates
intestinal chloride ion channels, thereby, increasing intestinal water secretion
and intestinal fluid chloride ion concentration. In basolateral membranepermeabilized
T84 gastrointestinal epithelial cells under chloride gradient conditions,
lubiprostone concentration-dependently increased short-circuit current
with an EC50 of approximately 20 nM. | | Originator | Sucampo (US) | | Uses | Lubiprostone (Ring Closed) is a bicyclic fatty acid used for the treatment of chronic constipation and constipation associated irritable bowel syndrome (IBS-C). | | Brand name | Amitiza | | General Description | This product is marketed as Amitiza bySucampo Pharmaceuticals, Inc. and Takeda PharmaceuticalsAmerica, Inc. to relieve chronic idiopathic constipation inadults. The recommended oral dosage is 24 μg 2 times a daywith food. Precautions and side effects are similar to thosefor other prostaglandin-derived products. | | Synthesis | Synthesis
of lupiprostone started with the tetrahydropyran (THP)
protected (-)Corey lactone 30. Desilylation
of 30 with TBAF in THF gave free carbinol in 82% yield
which was oxidized with oxalyl chloride and DMSO to give
corresponding crude aldehyde 31. Aldehyde 31 was condensed
with dimethyl 3,3,-difluoro-2-oxoheptylphosphonate
(32) in the presence of thallium ethoxide to give unsaturated
difluoroketone 33 which was hydrogenated with H2 over
Pd/C in ethyl acetate and the resulting ketone was subsequently
reduced with sodium borohydride in methanol to
give lactone 34 in excellent yield. The lactone 34 was reduced
to lactol 35 with DIBAL at -78??C in toluene and the
crude lactol 35 was condensed with 4-carboxybutyl triphenylphosphonium
bromide (36) in the presence of t-BuOK in
THF to yield compound 37. Crude 37 was reacted with benzyl
bromide and DBU in dichloromethane (DCM) to give the
benzyl ester in 96% yield. Oxidation of the alcohol with
Collins reagent and removal of the THP protecting group
under acidic conditions gave corresponding prostaglandin E2
benzyl ester 38. Finally, compound 38 was submitted to simultaneous
benzyl ester group cleavage and double bond
hydrogenation with H2 over Pd/C in ethyl acetate to give
lubiprostone (V) in 94% yield. 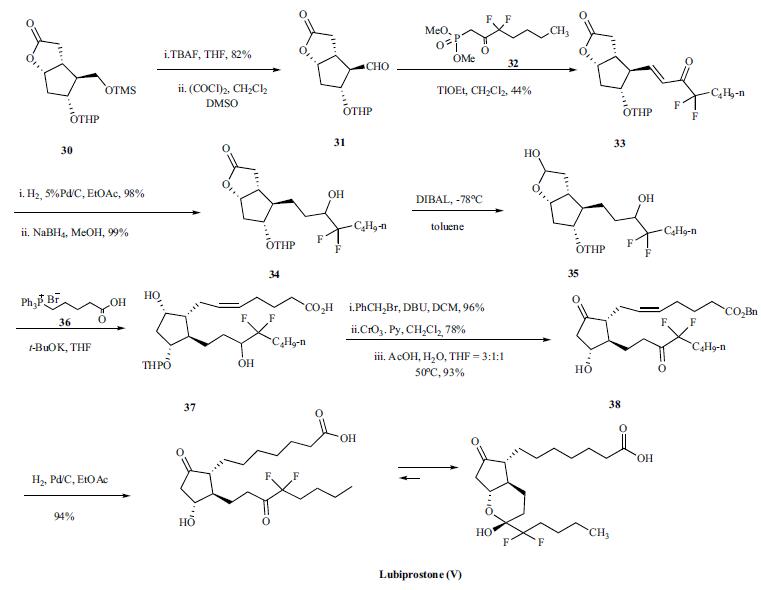
|
| | lubiprostone Preparation Products And Raw materials |
|