|
| | Methocarbamol Basic information |
| Product Name: | Methocarbamol | | Synonyms: | 1,2-Propanediol, 3-(2-methoxyphenoxy)-, 1-carbamate;1,2-Propanediol, 3-(o-methoxyphenoxy)-, 1-carbamate;1,2-Propanediol,3-(2-methoxyphenoxy)-,1-carbamate;2-Hydroxy-3-(2-methoxyphenoxy)propyl carbamate;2-Hydroxy-3-(o-methoxyphenoxy)propyl 1-carbamate;2-Hydroxy-3-(o-methoxyphenoxy)propyl carbamate;2-hydroxy-3-(o-methoxyphenoxy)propyl1-carbamate;2-propanediol,3-(2-methoxyphenoxy)-1-carbamate | | CAS: | 532-03-6 | | MF: | C11H15NO5 | | MW: | 241.24 | | EINECS: | 208-524-3 | | Product Categories: | ROBAXIN;Other APIs;Pharmaceuticals;Methocarbamol;API's;Active Pharmaceutical Ingredients;Organics;Amines;Aromatics;Intermediates & Fine Chemicals;532-03-6 | | Mol File: | 532-03-6.mol |  |
| | Methocarbamol Chemical Properties |
| Melting point | 95-97°C | | Boiling point | 384.01°C (rough estimate) | | density | 1.2611 (rough estimate) | | refractive index | 1.5080 (estimate) | | storage temp. | Sealed in dry,Room Temperature | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | neat | | pka | 13.08±0.20(Predicted) | | color | White to Off-White | | Merck | 14,5980 | | InChI | InChI=1S/C11H15NO5/c1-15-9-4-2-3-5-10(9)16-6-8(13)7-17-11(12)14/h2-5,8,13H,6-7H2,1H3,(H2,12,14) | | InChIKey | GNXFOGHNGIVQEH-UHFFFAOYSA-N | | SMILES | C(OC(=O)N)C(O)COC1=CC=CC=C1OC | | CAS DataBase Reference | 532-03-6(CAS DataBase Reference) | | NIST Chemistry Reference | Methocarbamol(532-03-6) | | EPA Substance Registry System | Methocarbamol (532-03-6) |
| | Methocarbamol Usage And Synthesis |
| Chemical Properties | White Solid | | Originator | Robaxin ,Robins,US,1957 | | Uses | Methocarbamol suppresses multisynaptic pathways in the spinal cord. It is used for relieving
spasms and skeletal muscle pain as well as for treating tetanus. Synonyms of this drug
are delaxin, forbaxin, robamol, robaxin, and tresortil. | | Uses | For use as an adjunct to rest, physical therapy, and other measures for the relief of discomforts associated with acute, painful musculoskeletal conditions. | | Uses | A muscle relaxant (skeletal). | | Definition | ChEBI: 2-hydroxy-3-(2-methoxyphenoxy)propyl carbamate is a carbamate ester that is glycerol in which one of the primary alcohol groups has been converted to its 2-methoxyphenyl ether while the other has been converted to the corresponding carbamate ester. It is a carbamate ester, a secondary alcohol and an aromatic ether. | | Manufacturing Process | The starting material for methocarbamol is 3-o-methoxyphenoxy-1,2- propanediol (guaiacol glyceryl ether) (see entry under Guaifenesin for its preparation). To a stirred suspension of 198.2 g (1.0 mol) of 3-omethoxyphenoxy-1,2-propanediol in 1,000 ml of dry benzene contained in a 5-liter, 3-neck, round bottom flask equipped with a thermometer, dropping funnel and blade stirrer, was added dropwise (in 30 minutes) a solution of 98.9 g (1.0 mol) of phosgene in 400 ml of cold dry benzene. The mixture was stirred at 30°C until all solid material dissolved (about 3 hours was required) and stirring was continued for 30 minutes longer. To this mixture was added
dropwise 79.1 g (1.0 mol) of dry pyridine, the temperature being held below
30°C by cooling. After addition of the pyridine, stirring at 30°C was continued
for 30 minutes.
The mixture was cooled to 7°C, extracted with two 500-cc portions of ice
water to remove pyridine hydrochloride, and the benzene solution of 3-omethoxyphenoxy-2-hydroxypropyl chlorocarbonate was added to 500 ml of
cold concentrated ammonium hydroxide. The mixture was vigorously stirred at
5°C for 6 hours, then the crude white precipitate of 3-o-methoxyphenoxy-2-
hydroxypropyl carbamate was filtered off, dissolved in 1,500 ml of hot
benzene and completely dried by codistillation of last traces of water with
benzene, treated with decolorizing carbon and filtered while hot. On cooling
160 g of product crystallized as white needles melting at 88° to 90°C. | | Brand name | Delaxin (Ferndale); Forbaxin
(Forest); Robaxin (Baxter Healthcare). | | Therapeutic Function | Muscle relaxant | | Synthesis Reference(s) | The Journal of Organic Chemistry, 22, p. 1595, 1957 DOI: 10.1021/jo01363a016 | | General Description | Methocarbamol, 3-(o-methoxyphenoxy)-1,2-propanediol 1-carbamate (Robaxin), issaid to be more sustained in effect than mephenesin. Likelysites for metabolic attack include the secondary hydroxylgroup and the two ring positions opposite the ether functions.The dihydric parent compound, guaifenesin, is used asan expectorant. | | Synthesis | Methocarbamol, 3-(2-methoxyphenoxy)-1,2-propanediol-1 carbamate
(15.3.13), is synthesized by successive reaction with phosgene and then ammonia into 3-
(2-methoxyphenoxy)propanediol-1,2. 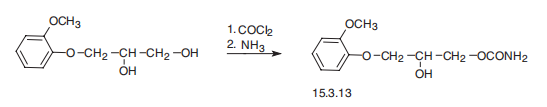
| | Veterinary Drugs and Treatments | In dogs and cats, methocarbamol is indicated (FDA approved) “as
adjunctive therapy of acute inflammatory and traumatic conditions
of the skeletal muscle and to reduce muscular spasms.” In horses,
intravenous use is indicated (FDA approved) “as adjunctive therapy
of acute inflammatory
and traumatic conditions of the skeletal
muscle to reduce muscular spasms, and effect striated
muscle relaxation.”
(Package insert; Robaxin?V—Robins) |
| | Methocarbamol Preparation Products And Raw materials |
|



