|
| | Chlorpropham Chemical Properties |
| Melting point | 41°C | | Boiling point | 247°C | | density | 1.18 | | refractive index | nD20 1.5388 | | Fp | 247°C | | storage temp. | 2-8°C | | solubility | Chloroform (Slightly), Methanol (Slightly) | | pka | 13.06±0.70(Predicted) | | form | crystalline | | color | light tan | | Odor | faint char. odor | | Water Solubility | 0.009 g/100 ml very poor | | Decomposition | 247 ºC | | Merck | 14,2187 | | BRN | 2211397 | | Exposure limits | An experimental carcinogen and neoplastigen | | Stability: | Stable. Incompatible with strong acids, strong bases, strong oxidizing agents. | | CAS DataBase Reference | 101-21-3(CAS DataBase Reference) | | NIST Chemistry Reference | Chlorpropham(101-21-3) | | IARC | 3 (Vol. 12, Sup 7) 1987 | | EPA Substance Registry System | Chlorpropham (101-21-3) |
| | Chlorpropham Usage And Synthesis |
| Physicochemical properties | A solid with low melting point of 41.4 ℃. The density is d30 1.180 and the refractive index is nD20 (after cooling) 1.5395.At 25 C, the solubility in the water is 89mg/L, and the degree of melting in the oil is medium (10% in the kerosene). It can be mixed with the lower alcohols, aromatics and most organic solvents. The purity of the industrial product is 98.5%, and the melting point is 38.5 ~ 40℃. Stable at lower than 100 C, but slowly hydrolyzed in acid and alkaline medium.
| | Toxicity | The acute oral administration of LD50 is 5 ~ 7.5g/kg in rats, and the acute oral administration is 5g/kg in rabbits. The drug had no toxic effect on rabbit skin for 20 hours or 2g/ kg feed for rats and dogs for 2 years. The acute oral administration is LD50>2g/kg for wild ducks. The TLm (48 hours) of goldfish and carp (48 hours) is 10 ~ 40mg/L. | | Mechanism of action | Mitotic inhibitor, in many perennial crops and some annual crops, it can be used alone or with other herbicides as pre-emergent selective weed removal. It is a plant growth regulator as well as a herbicide. It inhibits the activity of beta amylase, inhibits the synthesis of RNA and protein, interferes with oxidative phosphorylation and photosynthesis, and destroys cell division. Chloraniline is volatile. The steam can be absorbed by the bud in order to suppress weed bud growth. The residual effect in soil was longer than that of aniline, and the selectivity for some crops was smaller than that of aniline. In addition, it also regulates the growth of plants. It is used to inhibit the germination of potatoes during storage, flower and fruit thinning etc..
| | Applicable crops | Alfalfa, wheat, corn, soybean, sunflower, potato, sugar beet, rice, carrots, spinach, onions, etc.. | | Prevention and control object | Annual grasses and certain broadleaf weeds including ryegrass, barnyard grass, bluegrass, purslane, chickweed and Tusizi etc. annual grasses and certain broadleaf weeds.
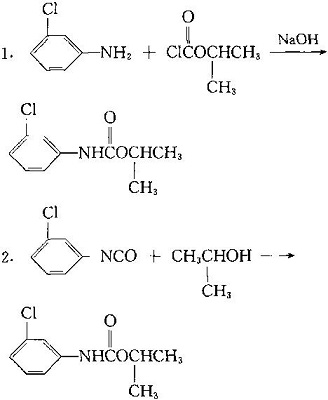
| | Usage method | The amount of effective components is 2.24 ~ 4.5kg per hectare per hectare below 16 C in pre seedling soil treatment. Double the amount above 24 centigrade and mix soil after application. The post seedling treatment is 1.2 ~ 3.5kg. For the post seedling treatment, the activity of herbicide is poor. But it can control amaranth and Polygonum, chickweed and purslane in the seedling. As a growth regulator, it is used to inhibit the germination of potatoes. | | Preparation method | It can be made by reaction of inter - Chloroaniline and isopropyl chloroformate or isopropanol and ISO - chlorophenyl isocyanate.
 | | Analysis method | Determine the CO2 produced by acidolysis.
Residue determination:
(1) The H2SO4 is hydrolyzed of 1:1 by the extraction of two hydrogen methane, and the alkali is obtained. The 3- hydrogen aniline is vaporized and the colorimetric determination is carried out by the hypochlorite - phenol method.
(2) Infrared spectroscopy.
| | Chemical Properties | beige to brown solid | | Uses | Herbicide; plant growth regulator. | | Uses | Preemergent and postemergent herbicide used to regulate plant growth and control
of weeds in carrot, onion, garlic and other crops | | Uses | Chlorpropham is particularly useful in agricultural settings. It is used in pesticide products for treatment of plants and soil. | | Definition | ChEBI: A carbamate ester that is the isopropyl ester of 3-chlorophenylcarbamic acid. | | General Description | Brown chunky solid. | | Air & Water Reactions | Insoluble in water. | | Reactivity Profile | Chlorpropham is a carbamate ester. Carbamates are chemically similar to, but more reactive than amides. Like amides they form polymers such as polyurethane resins. Carbamates are incompatible with strong acids and bases, and especially incompatible with strong reducing agents such as hydrides. Flammable gaseous hydrogen is produced by the combination of active metals or nitrides with carbamates. Strongly oxidizing acids, peroxides, and hydroperoxides are incompatible with carbamates. | | Hazard | Toxic by ingestion. | | Fire Hazard | Flash point data for Chlorpropham are not available, however Chlorpropham is probably combustible. | | Agricultural Uses | Herbicide, Plant growth regulator: Chlorpropham is a plant growth regulator that is used primarily in the U.S. to inhibit post-harvest potato sprouting. Other uses include pre-emergence control of grass weeds in alfalfa, beans, blueberries, cane berries, carrots, cranberries, ladino clover, garlic, seed grass, onions, spinach, sugar beets, tomatoes, safflower, soybeans, gladioli and woody nursery stock. It is used to control suckers in tobacco | | Trade name | ATLAS® CIPC 40; BEET-KLEEN® (with Fenuron® and isopropyl carbanilate); BUDNIP®; CAMPBELL’S® CIPC 40%; CHLORO IPC®; ELBANIL®; FASCO® WY-HOE; FURLOE®; FURLOE® 4EC; JACK WILSON® CHLORO 51 (OIL); LIRO METOXON®; MIRVALE®; MORCRAN® (with n-1-naphthylphthalamic acid); MSS® CICP; NEXOVAL®; PREVENOL® 56; PREVENTOL®; PREVENTOL® 56; PREWEED®; RESIDUREN®; RESIDUREN® EXTRA; SPROUT NIP®; SPROUT-NIP® EC; SPUDNIC®; SPUD-NIE®; STOPGERME®-S; TATERPEX®; TRIPEC® (with carbamic acid, phenyl-, 1-methylethyl ester); TRIHERBICIDE® CIPC; UNICROP® CIPC; WAREFOG®; Y3® | | Environmental Fate | Soil. Hydrolyzes in soil forming 3-chloroaniline (Bartha, 1971; Hartley and Kidd,
1987; Smith, 1988; Rajagopal et al., 1989). In soil, Pseudomonas striata Chester, a
Flavobacterium sp., an Agrobacterium sp. and an Achromobacter sp. readily degraded
chlorpropham to 3-chloroaniline and 2-propanol. Subsequent degradation by enzymatic
hydrolysis yielded carbon dioxide, chloride ions and unidentified compounds (Kaufman,
1967; Rajagopal et al., 1989). Hydrolysis products that may form in soil and in microbial
cultures include N-phenyl-3-chlorocarbamic acid, 3-chloroaniline, 2-amino-4-chlorophenol, monoisopropyl carbonate, 2-propanol, carbon dioxide and condensation products
(Rajagopal et al., 1989). The reported half-lives in soil at 15 and 29°C are 65 and 30 days,
respectively (Hartley and Kidd, 1987)
Plant. Chlorpropham is rapidly metabolized in plants (Ashton and Monaco, 1991).
Metabolites identified in soybean plants include isopropyl-N-4-hydroxy-3-chlorophenylcarbamate, 1-hydroxy-2-propyl-3′-chlorocarbanilate and isopropyl-N-5-chloro-2-hy
Photolytic. The photodegradation rate of chlorpropham in aqueous solution was
enhanced in the presence of a surfactant (TMN-10) (Tanaka et al., 1981). In a later study,
Tanaka et al. (1985) studied the photolysis of chlorpropham (50 mg/L) in aque
Chemical/Physical. Emits toxic phosgene fumes when heated to decomposition (Sax
and Lewis, 1987). In a 0.50 N sodium hydroxide solution at 20°C, chlorpropham was
hydrolyzed to aniline derivatives. The half-life of this reaction was 3.5 days (El-Dib and
Aly, 1976). Simple hydrolysis leads to the formation of 3-chlorophenylcarbamic acid and
2-propanol. The acid is very unstable and is spontaneously converted to 3-chloroaniline
and carbon dioxide (Still and Herrett, 1976) |
| | Chlorpropham Preparation Products And Raw materials |
|



