|
| | Trelagliptin succinate Basic information |
| | Trelagliptin succinate Chemical Properties |
| storage temp. | -20°C | | solubility | Soluble in DMSO | | form | Powder | | color | Off-white solid |
| | Trelagliptin succinate Usage And Synthesis |
| Description | Similar to omarigliptin,
trelagliptin succinate (XIX) is a highly selective, orally
delivered inhibitor of DPP-4 developed by Takeda Pharmaceuticals
and approved in Japan in March 2015 for the
treatment of type 2 DM. Interestingly, trelagliptin is
structurally similar to alogliptin, a DPP-4 inhibitor also
marketed by Takeda and described in our 2010 review,
differing only in the presence of a fluorine in the 5-position of
the cyanobenzyl moiety. Both trelagliptin and alogliptin are
potent inhibitors of DPP-4, with IC50s of 1.3 and 5.3 nM,
respectively. Notably, while similar drugs are dosed once
daily, trelagliptin is the first DPP-4 inhibitor approved for onceweekly
dosing. Kinetic analysis has revealed that trelagliptin is a
substrate-competitive, reversible, slow-binding inhibitor (t1/2 for
dissociation = ca. 30 min) of DPP-4, although the dissociation
time is insufficient to explain its long-acting effects. In a phase
III trial, once-weekly trelagliptin (100 mg) showed similar
efficacy and safety to once-daily alogliptin (25 mg) in patients
with type 2 DM inadequately controlled by diet and exercise.
The medicinal chemistry discovery of trelagliptin and
alogliptin as well as reviews of this class of compounds
have been published. | | Uses | Trelagliptin succinate (SYR-472) is a selective, long acting dipeptidyl peptidase-4 (DPP-4) inhibitor. An antidiabetic agent.
Orally active DPP-4 inhibitor that produces clinically and statistically significant improvements in glycaemic control in patients with type 2 diabetes. SYR472 has a long duration of action and is well tolerated in clinical studies. | | Clinical Use | Trelagliptin (SYR-472), a novel dipeptidyl peptidase-4 inhibitor used for the treatment of type 2 diabetes mellitus. Trelagliptin (as the salt Trelagliptin succinate) was approved for use in Japan in March 2015. Takeda, the company that developed Trelagliptin, chose to not get approval for the drug in the USA and EU. | | Synthesis | The kilogram-scale synthesis of trelagliptin succinate has
been reported in five steps from commercial starting material.102 Commercial 2-bromo-5-
fluorotoluene (162) was reacted with copper cyanide in
refluxing DMF to provide the corresponding nitrile in 60%
yield. Benzylic bromination with AIBN and 1,3-dibromo-5,5-
dimethylhydantoin (DBDMH) in DCE followed by treatment
with diethyl phosphite and DIPEA gave crude benzyl bromide
163, which was substituted directly with 6-chloro-3-methyluracil
(164) in the presence of DIPEA in NMP to provide
chloride 165 in 86% yield. Reaction with commercial (R)-3-
aminopiperidine dihydrochloride 166 in the presence of
K2CO3 and i-PrOH furnished trelagliptin as the freebase.
Conversion to the HCl salt and purification by crystallization
from dichloromethane, followed by a freebasing via 50%
NaOH, and treatment with succinic acid in THF/i-PrOH at 60
??C, and final recrystallization, generated trelagliptin succinate
XIX. 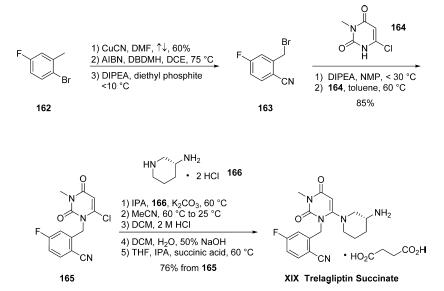
| | Research | Trelagliptin (Zafatek) is an orally active DPP-4 inhibitor developed by Takeda and approved in Japan for the treatment of type 2 diabetes mellitus (T2DM). Unlike other approved agents of its class, which are usually administered once daily, trelagliptin can be administered once weekly. Phase II development of trelagliptin was discontinued in the USA and EU, as Takeda considered that the costs associated with obtaining approval in these markets were prohibitive. |
| | Trelagliptin succinate Preparation Products And Raw materials |
|