|
| | Cabozantinib Malate Basic information |
| Product Name: | Cabozantinib Malate | | Synonyms: | 1-N-[4-(6,7-dimethoxyquinolin-4-yl)oxyphenyl]-1-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide,(2S)-2-hydroxybutanedioic acid;Smalate;XL184(S)-malate;N-(2-{[(3R)-1-(4-CHLOROBENZYL)-3-PYRROLIDINYL]AMINO}-2-OXOETHYL)-3-(TRIFLUOROMETHYL)BENZAMIDE HYDROCHLORIDE (1:1);Cabozantinib (XL184) S-malate;Cabozantinib malate, 98%, a potent VEGFR2 inhibitor;Carbozanitinb Malate;(2S)-2-Hydroxybutanedioic acid compd. with N-[4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-N'-(4-fluorophenyl)-1,1-cyclopropanedicarboxamide (1:1) | | CAS: | 1140909-48-3 | | MF: | C32H30FN3O10 | | MW: | 635.6 | | EINECS: | 691-711-0 | | Product Categories: | API;Inhibitors;1140909-48-3 | | Mol File: | 1140909-48-3.mol |  |
| | Cabozantinib Malate Chemical Properties |
| Melting point | 166-169°C | | storage temp. | Sealed in dry,Room Temperature | | solubility | DMSO (Slightly), Methanol (Slightly, Heated), Pyridine (Slightly) | | form | Solid | | color | White to Off-White |
| | Cabozantinib Malate Usage And Synthesis |
| Description | Cabozantinib S-MALATE,formerly known as XL184, trade name Cabometyx, is developed by Exelixis biopharmaceutical company in the United States. The drug is mainly targeted at MET and VEGFR2 tyrosine kinases that associated with the growth and proliferation of prostate cancer, inhibiting tumor metastasis and angiogenesis. Cabozantinib S-MALATE , the malate of Cabozantinib, is an effective VEGFR2 inhibitor, and IC50 is 0.035 nM. It also inhibits c-Met, Ret, Kit, Flt-1/3/4, Tie2 and AXL, and IC50 is 1.3 nM, 4 nM, 4.6 nM, 12 nM/11.3 nM/6 nM, 14.3 nM and 7 nM respectively.
Cabozantinib(Cometriq) was granted orphan drug status by the U.S. Food and Drug Administration (FDA) in January 2011. Cabozantinib(Cometriq) is approved by the U.S. FDA for medullary thyroid cancer. and advanced renal cell carcinoma in people who have received prior anti-angiogenic therapy. It is currently undergoing clinical trials for the treatment of prostate, bladder, ovarian, brain, melanoma, breast, non-small cell lung, pancreatic, and hepatocellular cancers. | | Chemical Properties | Cabozantinib (S)-malate salt is a white to off-white solid that is practically insoluble in aqueous media. | | Uses | Cabozantinib is a small molecule C-Met modulator. Cabozantinib acts as a potent multitargeted VEGFR2, Met, FLT3, Tie2, Kit and Ret inhibitor with IC50 of 0.035, 1.8, 14.4, 14.3 and 4.6 nM for VEGFR2,
Met, FLT3, Tie2 and Kit, respectively. Cabozantinib shows dose-dependent inhibition of tumor growth and tumor regression, associated with disruption of the tumor vasculature and extensive tumor cell a
poptosis. | | Uses | Cabozantinib malate is the malate of Cabozantinib, a potent VEGFR2 inhibitor with IC50 of 0.035 nM and also inhibits c-Met, Ret, Kit, Flt-1/3/4, Tie2, and AXL with IC50 of 1.3 nM, 4 nM, 4.6 nM, 12 nM/11.3 nM/6 nM, 14.3 nM and 7 nM, respectively. | | Definition | ChEBI: Cabozantinib malate is a malate salt that is the mono-(S)-malate salt of cabozantinib. A multi-tyrosine kinase inhibitor, used for the treatment of progressive, metastatic, medullary thyroid cancer. It has a role as a tyrosine kinase inhibitor, an antineoplastic agent and a prodrug. It contains a cabozantinib. | | Biological Activity | cabozantinib malate is a potent inhibitor of met andvegf receptor2 with ic50 values of 1.3nm and 0.035nm [1].cabozantinib is a pan-tyrosine kinase inhibitor and is developed as an oral treatment of various cancers including mtc, gbm, nsclc, pancreatic carcinoma, breast and colon cancer. the targets of cabozantinib are met, vegfr-2, ret, flt3, kit, axl as well as tek. in cellular assays, cabozantinib inhibits the phosphorylation of met, vegfr2, kit, flt3 and axl with ic50 values of 7.8, 1.9, 5.0, 7.5 and 42μm, respectively [1, 2].as a pan-tyrosine kinase inhibitor, cabozantinib can affect many biological processes. cabozantinib inhibits the tubule formation of hmvec cells with ic50 value of 6.7nm. in b16f10 cells, cabozantinib inhibits hgf-inducedmigration and invasion with ic50 values of 31nm and 9nm, respectively. moreover, cabozantinib shows anti-proliferation efficacy in a variety of tumors such as snu-5, hs746t, mda-mb-231 and u87mg. it is also reported that the combination of cabozantinib and gefitinib can cause potent inhibition of the gefitinib-resistant hcc827gr6 cell line [1, 2]. | | Clinical Use | Cabozantinib (S)-malate (Cometriq®), which was discovered and developed by Exelixis, gained
approval by the U.S. FDA in November 2012. The drug’s indication is for the treatment of medullary
thyroid cancer (MTC), and is the second drug for this disease after AstraZeneca’s vandetanib
(Caprelsa®). The drug was successfully launched on January 24, 2013. Cabozantinib inhibits
multiple receptor tyrosine kinases including RET, MET, VEGFR-1, -2 and -3, KIT, TRKB, FLT-3,
AXL, and TIE-2. It is currently also undergoing clinical trials for the treatment of prostate, ovarian,
brain, melanoma, breast, non-small cell lung, pancreatic, hepatocellular and kidney cancers. | | Synthesis | Of the
three syntheses of cabozantinib reported, the kilo-gram scale process route is described in the scheme. 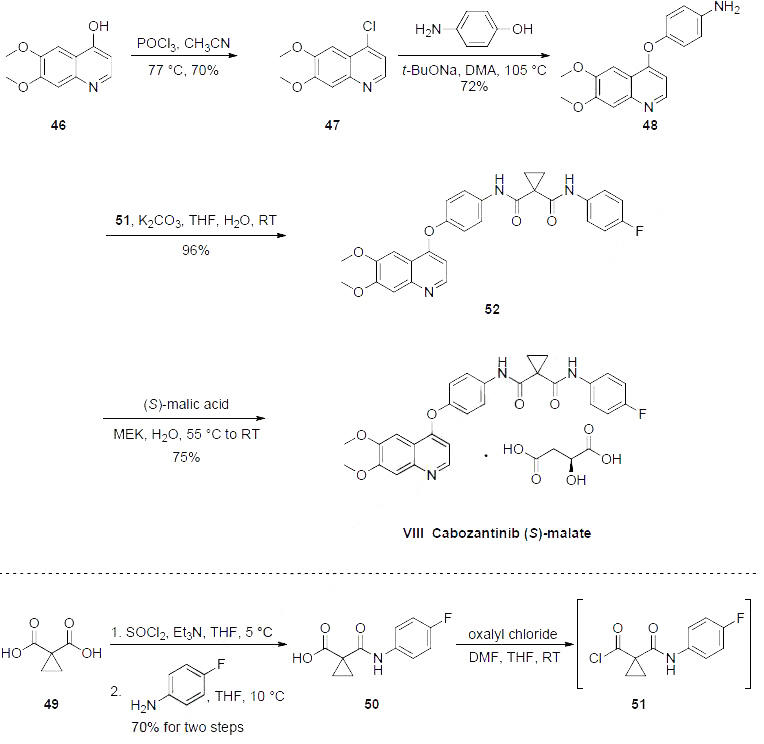
The preparation began with 6,7-dimethoxy-quinoline-4-ol (46) which upon treatment with POCl3
provided chloride 47 in 70% yield. Exposure of 47 to 4-aminophenol under basic conditions using t-
BuONa furnished diaryl ether 48 in 72% yield. This aniline was then coupled with amidoacid chloride
51 (which arose from the activation of commercial diacid 49 to the corresponding monochloride and
coupling with p-fluoroaniline and subsequent exposure to oxalyl chloride to furnish the transient acid
chloride) to construct cabozantinib as the free base 52 in 95% yield. Salt formation of cabozantinib 52
was carried out with (S)-malic acid, which ultimately delivered the final product of cabozantinib (S)-
malate (VIII) in 75% yield. | | Precautions | Cometriq has a boxed warning that increases the incidence of gastrointestinal perforation and fistula
Formation and severe bleeding. If you have severe stomach pain, or if you feel that you are suffocating and vomiting while eating or drinking, call your doctor. Warnings and precautions include thrombosis events, Wound complications, hypertension, jaw osteonecrosis, palm-toe erythema paresthesia Syndrome (PPES), proteinuria and reversible posterior leukoencephalopathy syndrome (RPLS). | | References | [1] yakes f m, chen j, tan j, et al. cabozantinib (xl184), a novel met and vegfr2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. molecular cancer therapeutics, 2011, 10(12): 2298-2308. DOI:10.1158/1535-7163.MCT-11-0264
[2] zhang y, guessous f, kofman a, et al. xl-184, a met, vegfr-2 and ret kinase inhibitor for the treatment of thyroid cancer, glioblastoma multiforme and nsclc. idrugs, 2010, 13(2): 112. PMCID:PMC3268517 |
| | Cabozantinib Malate Preparation Products And Raw materials |
|



