|
| | (2-MERCAPTOETHYL)TRIMETHYLSILANE Basic information |
| Product Name: | (2-MERCAPTOETHYL)TRIMETHYLSILANE | | Synonyms: | 2-(trimethylsilyl)-ethanethio;BEST;(2-MERCAPTOETHYL)TRIMETHYLSILANE;(2-Mercaptoethyl)trimethylsilane(2-Trimethylsilylethanethiol);2-(TRIMETHYLSILYL)ETHANETHIOL;ETHYLTHIOTRIMETHYLSILANE 97%;(2-Mercaptoethyl)trimethylsilane, BEST;2-TRIMETHYLSILANYL-ETHANETHIOL | | CAS: | 18143-30-1 | | MF: | C5H14SSi | | MW: | 134.32 | | EINECS: | | | Product Categories: | | | Mol File: | 18143-30-1.mol |  |
| | (2-MERCAPTOETHYL)TRIMETHYLSILANE Chemical Properties |
| Boiling point | 144-146 °C(lit.) | | density | 0.839 g/mL at 25 °C(lit.) | | refractive index | n20/D 1.454(lit.) | | Fp | 85 °F | | storage temp. | 2-8°C | | solubility | Soluble in MeOH, CH2Cl2, THF, and most organic
solvents. | | pka | 10.80±0.10(Predicted) | | BRN | 1732126 |
| Hazard Codes | Xi | | Risk Statements | 10-36/37/38 | | Safety Statements | 26-36 | | RIDADR | UN 1993 3/PG 3 | | WGK Germany | 3 | | RTECS | KJ2621000 | | F | 13 | | HazardClass | 3.2 | | PackingGroup | III | | Toxicity | mouse,LD50,intraperitoneal,2500mg/kg (2500mg/kg),Doklady Akademii Nauk SSSR. Proceedings of the Academy of Sciences of the USSR. For English translation, see DBIOAM and DKBSAS. Vol. 229, Pg. 1011, 1976. |
| | (2-MERCAPTOETHYL)TRIMETHYLSILANE Usage And Synthesis |
| Physical properties | bp 144–146 °C [52–54 °C (25 Torr)]; d 0.850. | | Uses | 2-(Trimethylsilyl)ethanethiol is a reagent used as a monoprotected sulfide dianion equivalent for
the two-step installation of a divalent sulfur atom into organic molecules. It participates in the reactions of Thiol Substitution Reactions, Source of Sulfur Acid Derivatives, etc. | | Preparation | The high commercial cost of
2-(trimethylsilyl)ethanethiol necessitates methods for its
lab scale synthesis. The radical addition of thiolacetic acid to
vinyltrimethylsilane occurs thermally to afford a 9:1 ratio of
regioisomers, the 1- and 2-(trimethylsilyl)ethyl thiolesters of
acetic acid (eq 1). Photochemical initiation may improve the
ratio of regioisomers obtained. Purification of the thiolacetate
by careful fractional distillation should be performed prior
to conversion to the thiol in order to ensure high purity. The
conversion to thiol is best performed with LAH3 or methanolic
K2CO3, but ammonolysis may also be suitable. Saponification
with KOH in water/ethanol is slightly less efficient.
Direct radical addition of liquid H2S to vinyl trimethylsilane
has been demonstrated, but the procedure is low-yielding and inconvenient. 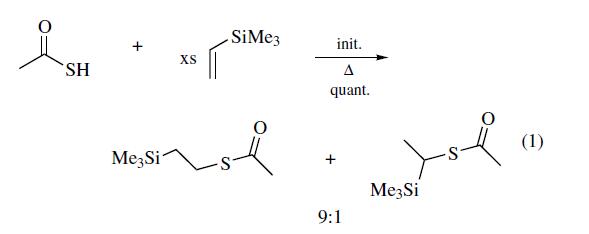
|
| | (2-MERCAPTOETHYL)TRIMETHYLSILANE Preparation Products And Raw materials |
|