| Description | N-Acetyl-L-cysteine is an N-acetylated derivative of cysteine, which contains a sulfhydryl group in the molecule, which can break the disulfide bond (-S-S-) of the mucin peptide bond, so that the mucin chain becomes a small molecule peptide chain, reducing The viscosity of mucin, so It is a dissolving drug for mucus in mucus, purulent sputum and respiratory tract. |
| Chemical Properties | N-Acetyl-L-cysteine is a white crystalline powder that has a garlic-like smell and sour taste. It is hygroscopic, and can dissolve in water or ethanol, but is insoluble in ether and chloroform. This substance is also acidic in aqueous solution, with a pH range of 2-2.75 for a concentration of 10g/L H2O. |
| Originator | Mucomyst ,Mead Johnson,US |
| Uses | A metabolite of Methyl Isocyanate.
N-Acetyl-L-cysteine, by itself a poor scavenger of oxidants, is converted inside cells to yield sulfane sulfur species, which are very potent scavengers of oxidants. |
| Uses | An antioxidant mucolytic acetylated amino acid.
N-acetyl-l-cysteine (NAC) is a derivative of the dietary amino acid l-cysteine. NAC has a high affinity for lung tissue, which it supports through mucolytic and antioxidant action. NAC also enhances glutathione production and plays a role in heavy metal detoxification.
|
| Definition | ChEBI: N-Acetyl-L-cysteine is an N-acetyl-L-amino acid that is the N-acetylated derivative of the natural amino acid L-cysteine. It is a pharma ceutical drug and nutritional supplement used primarily as a mucolytic agent and in the management of paracetamol (acetaminophen) overdose. Other uses include sulfate repletion in conditions, such as autism, where cysteine and related sulfur amino acids may be depleted. |
| Manufacturing Process | To a suspension of 35.2 grams (0.2 mol) of L-cysteine hydrochloride
monohydrate stirred in a reaction vessel containing 87 ml of 91% aqueous
tetrahydrofuran under a nitrogen atmosphere there is added 54.4 grams (0.4
mol) of sodium acetate trihydrate. The mixture is stirred for 20 minutes at
room temperature to insure neutralization of the hydrochloride salt resulting in
the formation of a suspension of equimolar amounts of cysteine and sodium
acetate.
The mixture is then chilled to 3-6°C by external cooling and 20 ml (20.8
grams, 0.21 mol) of acetic anhydride is added thereto in dropwise fashion
with cooling in the above range. The resulting mobile suspension is stirred for
6 hours at room temperature, allowed to stand overnight, and finally heated
at reflux (72°C) for 4 hours. The resulting suspension of sodium N-acetyl-Lcysteinate
is then neutralized by treatment at 5-10°C with 8 grams of
hydrogen chloride. Resulting sodium chloride is removed by filtration and the
product is isolated by distilling the solvent from the filtrate in vacuum and
crystallizing the residue from 35 ml of water, yield 26.3 grams (80.6%) of Nacetylcysteine
as a white solid, MP 109-110°C. |
| Brand name | Acetadote
(Cumberland); Mucomyst (Apothecon); Mucosil (Dey). |
| Therapeutic Function | Expectorant |
| benefits | n-acetyl-l-cysteine is a skin conditioner. It may also be used as an anti-aging ingredient given a demonstrated ability to regulate skin atrophy and reduce the appearance of fine lines and wrinkles. |
| General Description | N-Acetyl-L-cysteine is the N-acetyl derivative of the amino acid Lcysteine, and is a precursor in the formation of the antioxidant glutathione in the body. The thiol (sulfhydryl) group confers antioxidant effects and is able to reduce free radicals. This compound is sold as a dietary supplement commonly claiming antioxidant and liver protecting effects. It is used as a cough medicine because it breaks disulfide bonds in mucus and liquefies it, making it easier to cough up. It is also this action of breaking disulfide bonds that makes it useful in thinning the abnormally thick mucus in cystic and pulmonary fibrosis patients. In India it is marketed by Intas under the trade name 'Efetil'. |
| Biochem/physiol Actions | Antioxidant and mucolytic agent. Increases cellular pools of free radical scavengers. Reported to prevent apoptosis in neuronal cells but induce apoptosis in smooth muscle cells. Inhibits HIV replication. May serve as a substrate for microsomal glutathione transferase. |
| Mechanism of action | N-Acetyl-L-cysteine is a kind of membrane penetrating antioxidant. It has anti-inflammatory activity through regulating the activation of NF-KB and HIF-1α; as well as modulation of ROS. It can penetrate across the membrane, replenishes intracellular and glutathione and GSH to help the cell fight against oxidative stress. In addition to its antioxidant action, NAC acts as a vasodilator by facilitating the production and action of nitric oxide. This property is an important mechanism of action in the prophylaxis of contrast-induced nephropathy and the potentiation of nitrate-induced vasodilation (Millea 2009). |
| Side effects | At dosages of 1,200 mg twice daily or lower, N-acetylcysteine is well tolerated. At these dosages, side effects are unusual, but may include nausea, vomiting, diarrhea, transient skin rash, flushing, epigastric pain, and constipation.
www.aafp.org |
| Safety Profile | Poison by intraperitoneal route. Moderately toxic by other routes. Mutation data reported. When heated to decomposition it emits very toxic fumes of NO, and SOx, |
| Synthesis | Acetylcysteine, N-acetyl-L-cysteine (23.2.4), is synthesized by reacting
L-cysteine hydrochloride with acetic anhydride in the presence of sodium acetate. 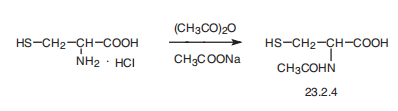
|
| Veterinary Drugs and Treatments | N-Acetyl-L-cysteine is a mucolytic agent that can be used to stop the melting effect of collagenases and proteases on the cornea. It is useful in halting melting through inhibition of metalloproteinases, but is not felt to be useful for melting caused by infectious agents. |
| Metabolism | N-Acetyl-L-cysteine undergoes transformation in the liver, and may be present in plasma as the parent compound or as various oxidised metabolites such as N-acetylcystine, N,N-diacetylcystine, and cysteine either free or bound to plasma proteins. Oral bioavailability is low (4-10%). It has been suggested that N-Acetyl-L-cysteine's low oral bioavailability may be due to metabolism in the gut wall and first-pass metabolism in the liver. |
| storage | Store at -20°C |
| References | http://www.sigmaaldrich.com/catalog/product/sigma/a0737?lang=en®ion=US
Grinberg, L, et al. "N-acetylcysteine amide, a novel cell-permeating thiol, restores cellular glutathione and protects human red blood cells from oxidative stress." Free Radical Biology & Medicine 38.1(2005):136-145.
Zhang, Xinsheng, et al. "N-Acetylcysteine Amide Protects Against Methamphetamine-Induced Oxidative Stress and Neurotoxicity in Immortalized Human Brain Endothelial Cells." Brain Research1275(2009):87-95.
Penugonda, S, et al. "Effects of N-acetylcysteine amide (NACA), a novel thiol antioxidant against glutamate-induced cytotoxicity in neuronal cell line PC12."Brain Research 1056.2(2005):132.
Lee, Kyung Sun, et al. "A novel thiol compound, N-acetylcysteine amide, attenuates allergic airway disease by regulating activation of NF-|[kappa]|B and hypoxia-inducible factor-1|[alpha]|." Experimental & Molecular Medicine 39.6(2007):756.
|



