|
| | 4'-Nitroacetanilide Basic information |
| | 4'-Nitroacetanilide Chemical Properties |
| Melting point | 213-215 °C(lit.) | | Boiling point | 312.97°C (rough estimate) | | density | 1.340 | | refractive index | 1.6180 (estimate) | | storage temp. | Store below +30°C. | | solubility | 2.2g/l | | pka | 13.91±0.70(Predicted) | | form | Solid | | color | Yellow to green-yellow or green-brown | | Water Solubility | 2.2g/L(room temperature) | | Merck | 14,6581 | | BRN | 2211962 | | Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. | | CAS DataBase Reference | 104-04-1(CAS DataBase Reference) | | EPA Substance Registry System | Acetamide, N-(4-nitrophenyl)- (104-04-1) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 3 | | RTECS | AE5075000 | | TSCA | Yes | | HS Code | 29242990 | | Toxicity | rat,LDLo,intraperitoneal,500mg/kg (500mg/kg),National Academy of Sciences, National Research Council, Chemical-Biological Coordination Center, Review. Vol. 5, Pg. 10, 1953. |
| | 4'-Nitroacetanilide Usage And Synthesis |
| Chemical Properties | solid | | Uses | 4-Nitroacetanilide was used as a test substrate and its hydrolysis was determined by UV spectroscopic measurements. It was also used to prepare 4-aminoacetanilide. | | Uses | Manufacture of nitraniline. | | Preparation | Preparation of 4'-Nitroacetanilide from acetanilide.
Principle: Aromatic compounds can be conveniently nitrated by the use of the nitrating mixture, which is normally a mixture of concentrated nitric acid and concentrated sulphuric acid. The function of sulphuric acid is to convert nitric acid into a highly reactive, electrophilic, nitronium ion, NO2+, which is the effective nitrating agent. Nitration of activated aromatic compounds is carried out under milder condition whereas deactivated rings require drastic conditions. Activating groups are o, p-directing, while deactivating groups are m-directing for electrophilic substitution.
Reaction:
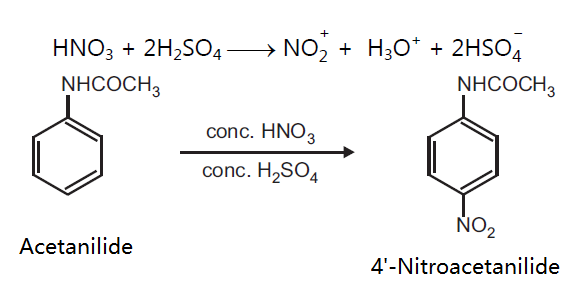
Procedure: Take 0.5 g acetanilide in 0.6 ml glacial acetic acid and add 1 ml conc. H2SO4 in a beaker. Cool the mixture in ice salt bath (5-10°C). To this add a mixture of 0.2 ml conc. H2SO4 and 0.25 ml conc. HNO3 dropwise. Maintain the temperature below 10°C during addition. After addition is over allow the mixture to attain room temperature and leave it for 30 minutes. Pour the mixture on to crushed ice (20 g) with stirring. Filter the separated product and wash with cold water. Dry the product, record the practical yield and re-crystallize it.
Re-crystallization: Dissolve the crude product in minimum amount of ethyl alcohol in a beaker by heating on a water bath. Filter the hot solution and cool the filtrate. The crystals of the product separate out. Filter, dry and record the melting point and TLC (using toluene as solvent).
| | Synthesis Reference(s) | Journal of the American Chemical Society, 75, p. 5905, 1953 DOI: 10.1021/ja01119a037
Tetrahedron Letters, 28, p. 1669, 1987 DOI: 10.1016/S0040-4039(00)95389-9 | | Purification Methods | Precipitate the anilide from 80% H2SO4 by adding ice, then wash with water, and crystallise from aqueous EtOH. Dry it in air. [Beilstein 12 IV 1632.] |
| | 4'-Nitroacetanilide Preparation Products And Raw materials |
|



