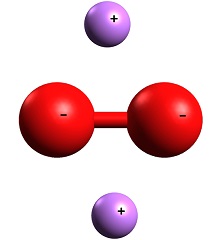
| Chemical Properties | Lithium peroxide, Li2O2, Mr 45.9, 2.36 g/cm3, has an active oxygen content of 34.8%, which is the highest of all metal peroxides. Lithium peroxide is pale yellow solid, stable at ambient temperature, and not hygroscopic. On heating to about 300 °C, it loses oxygen and forms lithium monoxide. |
| Chemical Properties | Lithium peroxide is a white crystalline solid or pale yellow powder which is thermodynamically stable at room temperature. The substance decomposes on heating to 195°C (the exact decomposition temperature is subject to dispute) with the formation of lithium oxide and oxygen. The decomposition forms the basis for one preparation of lithium oxide. High-purity lithium peroxide may be stored for long periods of time with no significant decomposition.
The reaction of carbon dioxide and lithium peroxide is noted above. When lithium peroxide is exposed to the air, lithium carbonate is the final product. Dissolving pure lithium peroxide in water produces an alkaline solution containing lithium ions and hydroperoxide ions. The decomposition of the solution is typical in that oxygen is released on heating or in the presence of a catalyst. The water solubility of lithium peroxide decreases with increasing temperature.
|
| Uses | Lithium peroxide is used in air purifiers. It is also used to produce high-purity oxygen. It acts as a catalyst for polymerization of styrene to polystyrene. Further, it serves as a curing agent for special polymers and a source of oxygen in sealed spaces such as submarines and in breathing apparatus. |
| Uses | At this time no important industrial uses of lithium peroxide are known. One interesting potential application is in the field of atmosphere regeneration for undersea and space applications, since the compound reacts with carbon dioxide to release oxygen: Li2O2+C02 -> Li2CO3 + 0.5O2. |
| Preparation | Lithium peroxide is prepared industrially by the reaction of lithium hydroxide monohydrate with hydrogen peroxide which yields lithium hydroperoxide monohydrate.
LiOH·H20 + H202 → LiOOH·H20+H20
The hydroperoxide may be dehydrated by heating in a vacuum to yield the peroxide.
2LiOOH·H20 → Li202+H202 + 2H20 |
| General Description | A white powder or sandy yellow granular solid. Irritates skin, eyes and mucous membranes. Used to produce a supply of high-purity oxygen. |
| Air & Water Reactions | Contact with water or moist air generates a large amount of heat and corrosive, alkaline lithium hydroxide [AAR 1991]. |
| Reactivity Profile | Lithium peroxide is strongly basic and an extremely powerful oxidizing agent. Accelerates the combustion of other materials, especially organic materials, involved in a fire. Can ignite wood, paper, oil, clothing, etc. on contact. May react explosively with hydrocarbons (fuels). Exposure to heat in a closed container may result in a vigorous reaction that violently ruptures the container. |
| Health Hazard | TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Fire may produce irritating and/or toxic gases. Toxic fumes or dust may accumulate in confined areas (basement, tanks, hopper/tank cars, etc.). Runoff from fire control or dilution water may cause pollution. |
| Fire Hazard | May explode from friction, heat or contamination. These substances will accelerate burning when involved in a fire. May ignite combustibles (wood, paper, oil, clothing, etc.). Some will react explosively with hydrocarbons (fuels). Containers may explode when heated. Runoff may create fire or explosion hazard. |
| Flammability and Explosibility | Notclassified |
| Safety Profile | A powerful oxidizer
and irritant to skin, eyes, and mucous
membranes. A very dangerous fire hazard
because it is an extremely powerful oxidizing
agent. Will react with water or steam to
produce heat; on contact with reducing
materials, can react vigorously. See also
LITHIUM COMPOUNDS, PEROXIDES,
and PEROXIDES, INORGANIC. |



