| Description | Triamterene is an inhibitor of the epithelial sodium channel (ENaC; IC50 = 4.5 μM for the rat channel). In vivo, triamterene (0.5-32 mg/animal) enhances sodium secretion and decreases potassium secretion in adrenalectomized rats. Formulations containing triamterene have been used in the treatment of edema. This product is also available as an analytical reference standard . |
| Chemical Properties | Yellow Solid |
| Originator | Jatropur,Rohm,W. Germany,1962 |
| Uses | This drug is recommended in combination with other diuretics for treating edema
caused by usual reasons such as circulatory insufficiency, cirrhosis of the liver, and
nephrotic syndrome. |
| Uses | Triamterene is a weak diuretic with potassium sparing properties; blocks Na+ reuptake in the kidneys.
|
| Uses | Triamterene is a potassium-sparing diuretic ie, it inhibits the urinary excretion of potassium |
| Definition | ChEBI: Triamterene is pteridine substituted at positions 2, 4 and 7 with amino groups and at position 6 with a phenyl group. A sodium channel blocker, it is used as a diuretic in the treatment of hypertension and oedema. It has a role as a diuretic and a sodium channel blocker. |
| Manufacturing Process | To a solution of 9 grams of 5-nitroso-2,4,6-triaminopyrimidine in 500 mi of
refluxing dimethylformamide is added 9 grams of phenylacetonitrile and the
refluxing is stopped. The 3 grams of anhydrous sodium methoxide is added
and the mixture is refluxed for 15 minutes. The mixture is chilled and the
solid is filtered and washed several times with warm water until the washings
are neutral. Drying gives yellow crystals which are recrystallized with a Darco
treatment from formamide-water heating the solution no hotter than 125°C.
This product is then suspended in filtered deionized water and warmed for 15
minutes. This yields the 2,4,7-triamino-6-phenylpteridine as yellow crystals
with a MP of 314° to 317°C. |
| Therapeutic Function | Diuretic |
| Biological Functions | Triamterene (Dyrenium)
results in changes in urinary electrolyte
patterns that are qualitatively similar to those produced
by spironolactone. The mechanism by which this
agents bring about the alterations in electrolyte loss,
however, is quite different. Triamterene
produces this effects whether or not aldosterone or any
other mineralocorticoid is present. The action of this
drug is clearly unrelated to endogenous mineralocorticoid
activity, and this drug is effective in adrenalectomized
patients. |
| General Description | Odorless yellow powder or crystalline solid. Melting point 316°C. Almost tasteless at first and with a slightly bitter aftertaste. Acidified solutions give a blue fluorescence. Used as a diuretic drug. |
| Air & Water Reactions | Sensitive to light; slowly oxidized upon exposure to air. Insoluble in water. |
| Reactivity Profile | 2,4,7-Triamino-6-phenylpteridine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. |
| Fire Hazard | Flash point data for 2,4,7-Triamino-6-phenylpteridine are not available; however, 2,4,7-Triamino-6-phenylpteridine is probably combustible. |
| Mechanism of action | Triamterene is a pyrazine derivative that inhibits reabsorption of sodium ions without
increasing excretion of potassium ions. It exhibits the same approximate effect as spironolactone;
however, it does not competitively bind with aldosterone receptors. Its action does
not have an effect on secretion of aldosterone or its antagonists, which are a result of direct
action on renal tubules.
This potassium sparing diuretic causes a moderate increase in excretion of sodium and
bicarbonate ions in urine, and it raises excretion of potassium and ammonia ions. It has little
effect on urine volume. |
| Clinical Use | Triamterene can be used in the treatment of congestive
heart failure, cirrhosis, and the edema caused by
secondary hyperaldosteronism. It is frequently used in
combination with other diuretics except spironolactone.
Amiloride, but not triamterene, possesses antihypertensive
effects that can add to those of the thiazides.
These K+-sparing diuretics have low efficacy when
used alone, since only a small amount of total Na reabsorption
occurs at more distal sites of the nephron.
These compounds are used primarily in combination
with other diuretics, such as the thiazides and loop diuretics,
to prevent or correct hypokalemia. The availability
of fixed-dose mixtures of thiazides with nonsteroidal
K+-sparing compounds has proved a rational
form of drug therapy. Both triamterene and amiloride
are available alone or in combination with hydrochlorothiazide. |
| Side effects | Because the actions of triamterene and amiloride
are independent of plasma aldosterone levels, their prolonged
administration is likely to result in hyperkalemia.
Both amiloride and triamterene are contraindicated
in patients with hyperkalemia.Triamterene
should not be given to patients with impaired renal
function. Potassium intake must be reduced, especially
in outpatients.A folic acid deficiency has been reported
to occur occasionally following the use of triamterene. |
| Synthesis | Triamterene, 2,4,7-triamino-6-phenylpteridine (21.5.13), is synthesized in by
the following scheme. Reacting guanidine with malonodinitrile gives 2,4,6-triaminopyrimidine
(21.5.11). This undergoes nitrosation by reacting it with nitric acid, which results in the formation of 5-nitroso-2,4,6-triaminopyrimidine (21.5.12), which upon condensation with
benzyl cyanide in the presence of sodium methoxide cyclizes into triamterene (21.5.13). 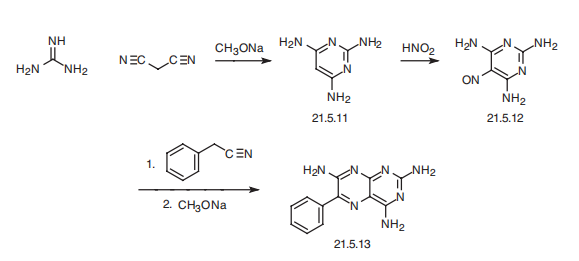
|
| Veterinary Drugs and Treatments | Triamterene is a potassium-sparing diuretic that potentially could
be used as an alternative to spironolactone for the adjunctive treatment
of congestive heart failure in dogs, however, there is little experience
associated with its use in dogs or cats. |
| Drug interactions | Potentially hazardous interactions with other drugs
ACE inhibitors and angiotensin-II antagonists:
enhanced hypotensive effect (risk of severe
hyperkalaemia).
Analgesics: increased risk of nephrotoxicity with
NSAIDs; increased risk of hyperkalaemia, especially
with indometacin; antagonism of hypotensive effect.
Antibacterials: avoid concomitant use with
lymecycline.
Antidepressants: enhanced hypotensive effect with
MAOIs; increased risk of postural hypotension with
tricyclics.
Antipsychotics: enhanced hypotensive effect with
phenothiazines.
Antihypertensives: enhanced hypotensive effect;
increased risk of first dose hypotensive effect of postsynaptic alpha-blockers, e.g. prazosin.
Ciclosporin: increased risk of hyperkalaemia.
Cytotoxics: increased risk of nephrotoxicity and
ototoxicity with platinum compounds.
Lithium: reduced excretion of lithium (risk of
lithium toxicity).
Potassium salts: increased risk of hyperkalaemia.
Tacrolimus: increased risk of hyperkalaemia. |
| Metabolism | Triamterene is extensively metabolised apparently via the
cytochrome P450 isoenzyme CYP1A2.
It is mainly excreted in the urine in the form of
metabolites with some unchanged triamterene; variable
amounts are also excreted in the bile. |



